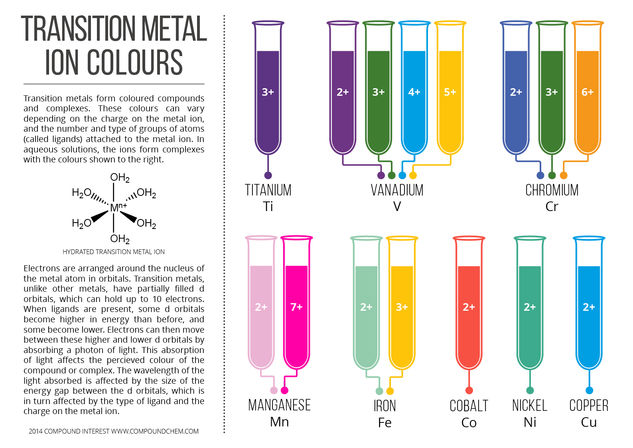
© Compound InterestColours of Transition Metal Ions in Aqueous Solution
This graphic looks at the colours of transition metal ions when they are in aqueous solution (in water), and also looks at the reason why we see coloured compounds and complexes for transition metals. This helps explain, for example, why rust (iron oxide) is an orange colour, and why the Statue of Liberty, made of copper, is no longer the shiny, metallic orange of copper, but a pale green colour given by the compound copper carbonate.
The colour can be affected by several variables. Different transition metals will exhibit different colours; as shown in the graphic above, different charges on the same transition metal can also accomplish this. The ligand also has an effect, and the same charge metal ion can be differently coloured depending on the ligands that are bound to it.